What Is Noble Gas Configuration
What are Noble Gases?
Noble gases are the elements present in group-18 of the modernistic periodic table. Their valence trounce configuration is full, due to which they are chemically inert. They are also known as inert gases and aerogens. Group-xviii belongs to the p-block in the modern periodic table.

They have very low chemic reactivity considering of their stable electronic configuration. Considering of this, they exercise non form molecules and are found as monoatomic gases. At normal room temperature and force per unit area, they are in a gaseous land.
Elements of grouping-18: Helium, Neon, Argon, Krypton, Xenon, Radon
Full general electronic configuration: [inert gas] nsiinp6 (exception He: 1s2)
Download the PDF of Noble Gases
Backdrop of Noble Gases
- Noble gases are found in the monoatomic grade at room temperature and pressure.
- The diminutive radius increases with diminutive number every bit we go down in the grouping.
- These elements have low melting and boiling points.
- They do not possess any taste, color, or odor.
- They are sparingly soluble in water.
- Due to their stable electronic configuration, they are mostly chemically unreactive.
Properties of Helium
Of all the substances, Helium is found to have the lowest boiling point. Information technology is a light and non-inflammable gas and is used in balloons for meteorological purposes. It is also used for experimental purposes, in nuclear reactors, and for clinical purposes. In pocket-sized concentrations, helium is non-toxic. Information technology is too the lightest element of group 0.
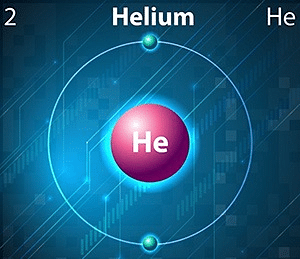
Moreover, Helium is an south-block chemical element that corresponds to period ane and grouping 18 of the modern periodic table. The electron configuration of helium is 1s2 which is why this element has a total of 2 electrons in its valence shell. The density of this element corresponds to ane.7186 grams per litre under standard conditions for temperature and pressure. Just in its liquid land, the density becomes approximately equal to 0.145 grams per cube centimetre.
Diminutive Symbol: He
Atomic Number: 2
Electronic Configuration: 1s2
Menstruation: 1
Valence electrons: ii
Backdrop of Neon
Neon is the 2d-lightest element of group 0. It is used in calorie-free bulbs and making advertizement signs. Although under standard atmospheric condition for temperature and force per unit area (STP), it is colourless under normal weather, it turns cherry-orangish in a vacuum belch tube. Information technology is also used equally a cryogenic refrigerant. This gas does not possess whatever characteristic odor.
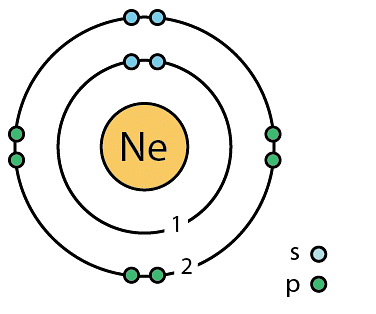
Moreover, neon is the 2d-lightest element of group 0 after helium. The humid betoken of neon is 27.104 Kelvin (or -246.046 degrees Celsius). At STP, the density of this gas is approximately equal to 0.9 grams per litre. However, in its liquid state i.due east, at a temperature equal to its boiling betoken, the density of neon is equal to ane.207 grams per cubic centimeter.
Atomic Symbol: Ne
Atomic Number: ten
Electronic Configuration: [He] 2sii2p6
Flow: 2
Valence electrons: 8
Properties of Argon
Later nitrogen and oxygen, argon is the third nigh abundant gas in the temper with a concentration of 0.93%. Argon is oft used to create an inert atmosphere in industrial processes. Ane such use is for the production of titanium and other reactive elements.
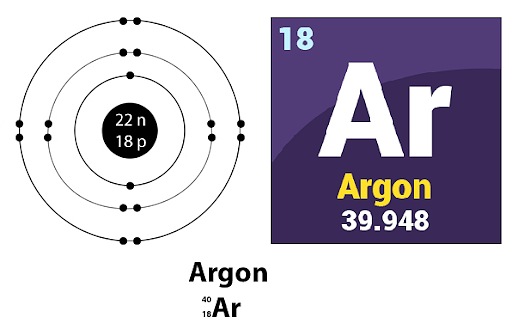
Nether standard conditions for temperature and pressure, argon exists equally a colorless gas that displays a violet or lilac-colored glow when it is placed in an electric field.
Moreover, Argon is a p-block element that is equal to group xviii and period three of the modern periodic table. The melting point of argon is 83.81 Kelvin while its boiling point corresponds to 87.302 Kelvin and under the STP, the density of argon is approximately equal to i.784 grams per litre.
Atomic Symbol: Ar
Atomic Number: 18
Electronic Configuration: [Ne] 3s23p6
Catamenia: 3
Valence electrons: viii
Properties of Krypton
Krypton is among the rarest gases plant on Earth. It is used in fluorescent lights. One of its isotopes, 85Kr, is used in the medical field. It is not completely unreactive like other noble gases and reacts with chlorine gas and the gas is also known to exist tasteless.
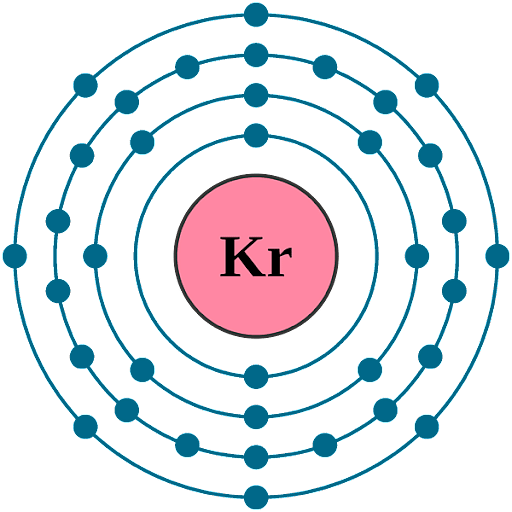
The melting point of this chemical element corresponds to 115.78 Kelvin (approximately) whereas the boiling point of this chemical element corresponds to 119.three Kelvin. Nether the standard temperature and pressure level, the density of this chemical element is roughly equal to three.75 grams per litre.
Diminutive Symbol: Kr
Atomic Number: 36
Electronic Configuration: [Ar] 3d104sii4p6
Period: 4
Valence electrons: 8
Properties of Xenon
Xenon is a heavy and rare noble gas. It is iv.5 times heavier than air. In the medical field, it is used every bit an anesthetic agent. Information technology has applications in photography where it is used in high-speed flashbulbs. Different other inert gases, Xenon is known to form some chemical compounds.
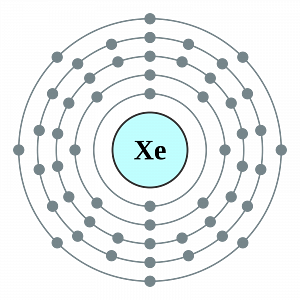
Under standard weather, this element exists as a monoatomic gas that is colorless as well as odorless. The melting indicate of xenon corresponds to 161.four Kelvin whereas the boiling bespeak of this element corresponds to 165.05 Kelvin. Under the standard temperature and pressure, the density of xenon is approximately equal to 5.89 grams per litre.
Diminutive Symbol: Xe
Atomic Number: 54
Electronic Configuration: [Kr] 4dten5sii5p6
Period: 5
Valence electrons: eight
Properties of Radon
Radon is a radioactive gas and is not found directly in the atmosphere. Information technology is 1 of the rarest elements of the group. 222Rn is the nearly stable isotope of radon and has a half-life of iii.viii days. 1 of the prominent uses of radon is in cancer therapy.
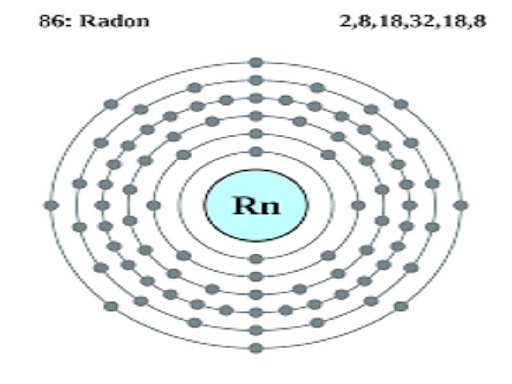
Under standard conditions of temperature and force per unit area, radon, like nearly other noble gases, is a colorless and odorless gas and does non have any feature gustation. The melting and boiling points of radon correspond to 202 Kelvin and 211.5 Kelvin respectively.
Atomic Symbol: Rn
Atomic Number: 86
Electronic Configuration: [Xe] 4f145dten6sii6phalf dozen
Menses: 6
Valence electrons: viii
Recommended video:
Things to Remember
- Group-18 elements are more often than not chemically unreactive because of their stable electronic configuration and thus are known as noble gases or inert gases.
- Their general electronic configuration is of the type [inert gas] ns2np6, except for Helium, whose configuration is 1s2.
- Nether normal weather of room temperature and pressure, group-18 elements are found in gaseous form. They are found in monoatomic forms and are colourless, odorless, and tasteless.
- Helium has the lowest humid point of all substances.
- Neon turns reddish-orange in a vacuum belch tube but is otherwise colorless under normal conditions.
- Argon has the third-highest concentration of all gases on earth.
- Xenon was the showtime inert gas institute to react to form chemical compounds.
- Radon is radioactive in nature and is used in cancer therapy.
Sample Questions
Ques: Why are group-18 elements known as noble gases? (2 marks)
Ans: The valence shell configuration of group-18 elements is full due to which they are mostly chemically unreactive in nature. And are hence known equally noble gases or inert gases.
Ques: What are the uses of Helium? (3 marks)
Ans: Uses of Helium:
- Information technology is used for creating an inert atmosphere to make fiber optics, semiconductors and for arc welding.
- As information technology diffuses quickly, information technology is used in auto air-workout to observe leaks.
- One of the prominent uses of Helium these days is for cryogenic research.
- Information technology is used as a breathing mix for deep-sea diving as it does not have whatsoever narcotic effects.
- It is also used in space shuttles and nuclear reactors.
Ques: List out the backdrop of noble gases. (five marks)
Ans: Properties of noble gases
- They are found in a gaseous country at normal temperature and pressure level and are colorless, odorless, and tasteless.
- Equally their valence trounce is full, they are stable and are more often than not chemically unreactive. Hence, they are found in the monoatomic form.
- They take low melting and boiling points.
- They have loftier ionization enthalpy because of their stable electronic configuration.
- Noble gases are sparingly soluble in water.
- As we go down in the grouping, their atomic radius increases with the diminutive number.
Ques: Which of the following noble gas southward is least polarized: (1 mark)
- Ar
- Xe
- Ne
- He
Ans: The right pick is d. He is the only noble gas s which is least polarized considering of its minor size.
Ques: Which of the following element of group 0 does non possess octate of due east- in the outermost trounce: (1 mark)
- Ne
- Rn
- Ar
- He
Ans: The correct option is a. Ne which is noble gas that does not possess octate of e- in the outermost shell.
Ques: What is the valency of inert gases? (2 marks)
- 2
- viii
- 0
- 1
Ans: Inert gases have 8 electrons in their valence crush, except Helium. Helium has 2 electrons. This makes their outermost orbit completely full and gives them a stable configuration. Hence, their valency is zippo.
Ques: Which inert gas does not follow the ns ii np 6 electron configuration? (one marker)
- Helium
- Krypton
- Xenon
- Argon
Ans: All inert gases have an ns2np6 electron configuration except Helium, whose electronic configuration is 1s2.
Ques: Which mixture is used for breathing by deep-ocean divers? (ii marks)
- Oxygen and neon
- Oxygen and hydrogen
- Oxygen and nitrogen
- Oxygen and helium
Ans: Helium is used with oxygen for breathing past deep-sea divers because it does not have a narcotic effect and helps them to think clearly.
Ques: Write the properties of Argon. (iii marks)
Ans: After nitrogen and oxygen, argon is the tertiary most abundant gas in the atmosphere with a concentration of 0.93%. Argon is often used to create an inert atmosphere in industrial processes. One such utilize is for the production of titanium and other reactive elements. Under standard conditions for temperature and pressure, argon exists as a colorless gas that displays a violet or lilac-colored glow when information technology is placed in an electric field.
Moreover, Argon is a p-cake element that is equal to group 18 and period 3 of the mod periodic table. The melting point of argon is 83.81 Kelvin while its boiling point corresponds to 87.302 Kelvin and under the STP, the density of argon is approximately equal to i.784 grams per litre.
Ques: What are the physical properties of Neon? (3 marks)
Ans: Under standard weather for temperature and pressure (STP), it is colourless under normal atmospheric condition, information technology turns reddish-orange in a vacuum belch tube. It is also used as a cryogenic refrigerant. This gas does not possess any characteristic odor. Moreover, neon is the second-lightest noble gas after helium. It is as well nontoxic but can act as a simple asphyxiant.
Ques: Write the physical and chemical backdrop of Radon. (3 marks)
Ans: Radon is a radioactive gas and is not establish directly in the atmosphere. Information technology is 1 of the rarest elements of the group. 222Rn is the most stable isotope of radon and has a half-life of iii.8 days. Ane of the prominent uses of radon is in cancer therapy.
Nether standard conditions of temperature and pressure, radon, similar most other noble gases, is a colorless and odorless gas and does not have any characteristic taste. The melting and boiling points of radon represent to 202 Kelvin and 211.five Kelvin respectively.
Atomic Symbol: Rn
Diminutive Number: 86
Electronic Configuration: [Xe] 4fxiv5d106s26p6
Catamenia: 6
Valence electrons: viii
What Is Noble Gas Configuration,
Source: https://collegedunia.com/exams/what-are-noble-gases-configuration-physical-chemical-properties-science-articleid-761
Posted by: morelandshrem1977.blogspot.com


0 Response to "What Is Noble Gas Configuration"
Post a Comment